Dr. Melissa C. Srougi
Research Areas:

- Experimental targeted cancer chemotherapies
- Breast cancer
- DNA damage and repair
- Redox signaling
- Transcriptional regulation
Techniques:
- Mammalian cell culture
- Dose-response assays
- Flow cytometry
- SDS-PAGE
- Western blot
- Immunoprecipitation assays
- Redox signaling
- Fluorescence microscopy
- RNA-seq data analysis
- Molecular cloning and transfection
- Comet assays
Project Description:
The long-term goal of our research is to exploit natural products that target breast cancers for the advancement of precision medicine with notable clinical benefit. β-Lapachone (β-lap) is a naturally occurring antitumor quinone currently in Phase I clinical trials that is selectively bioactivated in tumors expressing NAD(P)H:quinone oxidoreductase 1 (NQO1). NQO1 is constitutively overexpressed in a number of solid tumors including breast (~60%) (1-3), non-small cell lung carcinomas (NSCLCs) (>85%), and pancreatic cancers (~85%) compared to associated normal tissues (4-7). Interestingly, breast tumors with mutations in breast cancer susceptibility genes BRCA1/2 also overexpress NQO1. However, the efficacy of β-lap has not been tested in these or other tumors with inherent defects in DNA repair.
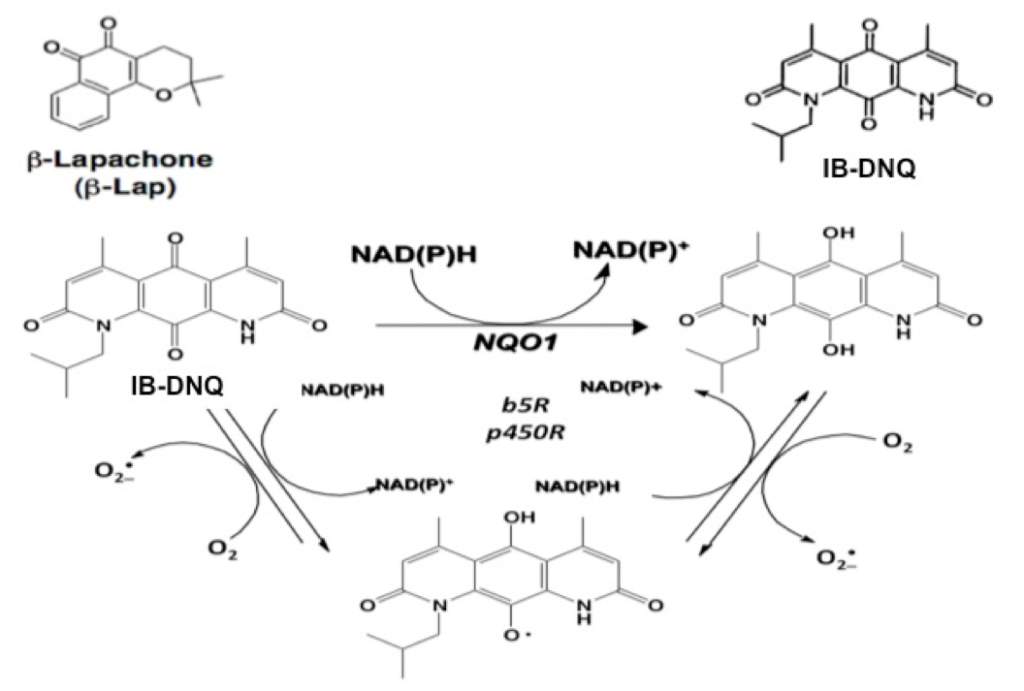
Figure 1. NQO1 futile redox cycling of β-lap or IB-DNQ.
NQO1-mediated futile cycle for IB-DNQ is shown.
NQO1 metabolizes β-lap via a two-electron oxidoreduction, resulting in the generation of an unstable hydroquinone form that rapidly reverts back to the parent quinone, causing multiple rounds of redox cycling (Fig. 1). β-Lap-induced reactive oxygen species (ROS) cause DNA base damage and DNA breaks (both single (SSB) and double-stranded (DSB)(8). SSBs and cytosolic Ca2+ release from endoplasmic reticulum pools hyperactive poly(ADP-ribose) polymerase-1 (PARP-1) resulting in cell death due to NAD+ and ATP loss (8-10). Interestingly, sub-lethal doses of β-lap, in combination with DNA damaging agents such as ionizing radiation, result in tumor sensitization (11,12). Recently, a novel more potent derivative of β-lap, isobutyldeoxynyboquinone (IB-DNQ) has been generated, which kills NQO1+ cells in a manner similar to β-lap (13). We hypothesize that sub-lethal doses of β-lap or IB-DNQ will induce low-level DNA damage in NQO1+; BRCA1/2-mutant breast tumors. Existing deficits in DNA repair caused by BRCA1/2 mutations in these cells will, therefore, selectively sensitize them to sub-lethal doses of β-lap- or IB-DNQ resulting in lethality. Furthermore, combination treatment with PARP inhibitors (PARPi) will further potentiate β-lap- or IB-DNQ- induced lethality for genotype-driven cytotoxicity, and thereby, circumvent possible resistance mechanisms. Two projects will be pursued to test this hypothesis:
- To determine the mechanism of β-lap or IB-DNQ-induced cell death in NQO1+;BRCA1/2-mutant breast cancers.
- To elucidate the efficacy of β-lap or IB-DNQ treatment in NQO1+;BRCA1/2-mutant breast cancers with or without PARPi combination treatment.
The findings gleaned from our studies will provide insight into the mechanisms of precision targeting of β-lap or IB-DNQ in NQO1-expressing BRCA1/2 mutant breast tumors to increase tumor response to therapy while decreasing normal tissue toxicity.
References
- Siegel, D., and Ross, D. (2000) Immunodetection of NAD(P)H:quinone oxidoreductase 1 (NQO1) in human tissues. Free Radic Biol Med 29, 246-253
- Yang, Y., Zhang, Y., Wu, Q., Cui, X., Lin, Z., Liu, S., and Chen, L. (2014) Clinical implications of high NQO1 expression in breast cancers. J Exp Clin Cancer Res 33, 14
- Menzel, H. J., Sarmanova, J., Soucek, P., Berberich, R., Grunewald, K., Haun, M., and Kraft, H. G. (2004) Association of NQO1 polymorphism with spontaneous breast cancer in two independent populations. Br J Cancer 90, 1989-1994
- Dong, Y., Bey, E. A., Li, L. S., Kabbani, W., Yan, J., Xie, X. J., Hsieh, J. T., Gao, J., and Boothman, D.A. (2010) Prostate cancer radiosensitization through poly(ADP-Ribose) polymerase-1 hyperactivation. Cancer Res 70, 8088-8096
- Dong, Y., Chin, S. F., Blanco, E., Bey, E. A., Kabbani, W., Xie, X. J., Bornmann, W. G., Boothman, D. A., and Gao, J. (2009) Intratumoral delivery of beta-lapachone via polymer implants for prostate cancer therapy. Clin Cancer Res 15, 131-139
- Lewis, A. M., Ough, M., Hinkhouse, M. M., Tsao, M. S., Oberley, L. W., and Cullen, J. J. (2005) Targeting NAD(P)H:quinone oxidoreductase (NQO1) in pancreatic cancer. Mol Carcinog 43, 215- 224
- Marin, A., Lopez de Cerain, A., Hamilton, E., Lewis, A. D., Martinez-Penuela, J. M., Idoate, M. A., and Bello, J. (1997) DT-diaphorase and cytochrome B5 reductase in human lung and breast tumours. Br J Cancer 76, 923-929
- Bentle, M. S., Reinicke, K. E., Bey, E. A., Spitz, D. R., and Boothman, D. A. (2006) Calcium- dependent modulation of poly(ADP-ribose) polymerase-1 alters cellular metabolism and DNA repair. J Biol Chem 281, 33684-33696
- Bey, E. A., Bentle, M. S., Reinicke, K. E., Dong, Y., Yang, C. R., Girard, L., Minna, J. D., Bornmann, W. G., Gao, J., and Boothman, D. A. (2007) An NQO1- and PARP-1-mediated cell death pathway induced in non-small-cell lung cancer cells by beta-lapachone. Proc Natl Acad Sci U S A 104, 11832-11837
- Tagliarino, C., Pink, J. J., Dubyak, G. R., Nieminen, A. L., and Boothman, D. A. (2001) Calcium is a key signaling molecule in beta-lapachone-mediated cell death. J Biol Chem 276, 19150-19159
- Boothman, D. A., Greer, S., and Pardee, A. B. (1987) Potentiation of halogenated pyrimidine radiosensitizers in human carcinoma cells by beta-lapachone (3,4-dihydro-2,2-dimethyl-2H- naphtho[1,2-b]pyran- 5,6-dione), a novel DNA repair inhibitor. Cancer Res 47, 5361-5366
- Boothman, D. A., Trask, D. K., and Pardee, A. B. (1989) Inhibition of potentially lethal DNA damage repair in human tumor cells by beta-lapachone, an activator of topoisomerase I. Cancer Res 49, 605-612